By Robert Spénard
Fuel cells are not a new invention. The first fuel cells were invented by Sir William Grove in 1838. It was a cell with two structures which were fed by either hydrogen or oxygen named the cell the Gas Voltaic Battery, which produced very little electricity. The fact that he created an electricity-producing device in 1832 fifty years before Edison commercialized electricity is something worth noting. Then in 1932 Francis Thomas Bacon, an English engineer took Grove’s work to a new level when he invented the first commercial hydrogen-oxygen fuel cell; which he called the alkaline fuel cell. The cell was so successful that NASA has been using it in satellites, spacecraft and stations.
Wikipedia defines a hydrogen fuel cell as;
A fuel cell is an electrochemical cell that converts the chemical energy of a fuel (often hydrogen) and an oxidizing agent (often oxygen) into electricity through a pair of redox reactions. Fuel cell are different from most batteries in requiring a continuous source of fuel and oxygen (usually from air) to sustain the chemical reaction, whereas in a battery the chemical energy usually comes from metals and their ions or oxides that are commonly present in the battery, except in flow batteries. Fuel cells can produce electricity continuously for as long as fuel and oxygen are supplied.
The best explanation of how a hydrogen fuel cell works can be found on the website for the Canadian Hydrogen and Fuel Association’s (CHFCA) website and is posted here.
[1]Fuel cells can deliver the zero-pollution, high-efficiency answer to much of our air pollution and global warming dilemmas.
A fuel cell is an electrochemical power generation device that combines hydrogen fuel, with oxygen from air, to produce electricity, with water and heat as the only by-products.
Fuel cells offer a variety of benefits compared to traditional power generation – they are more fuel-efficient, operate with very little noise and produce no harmful emissions at point of use.
Fuel cells and batteries are similar as they both generate electricity. But a battery stores energy in its electrodes, while a fuel cell uses an external fuel such as hydrogen allowing it to continue operating as long as fuel is available. Unlike conventional batteries however, fuel cells do not contain harmful materials, nor do they have moving parts thereby minimizing maintenance requirements.
Fuel cells have a number of additional advantages, including:
- High efficiency
- Quick refuelling
- Quiet operation
- Low vibration
- Compact size
- Rapid response to changes in electrical demand
- Low maintenance costs
- Long lifetimes
- Flexibility in installation and operation
- Longer ranges, increasingly important for larger, heavier vehicles.
All fuel cells have a similar configuration, an electrolyte and two electrodes, but there are different types of fuel cells based mainly on what electrolyte they use. There are six main types of fuel cells – PEM (proton exchange membrane), DMFC (direct methanol fuel cell), MCFC (molton carbonate fuel cell), PAFC (phosphoric acid fuel cell), SOFC (solid oxide fuel cell) and AFC (alkaline fuel cell). The dominant technology is the proton exchange membrane fuel cell due to its versatility, durability, and use for a range of applications.
A fuel cell “stack” is made up of single fuel cells layered together. Depending on the application, the fuel cell stack may contain hundreds of individual cells layered together – this scalability allows fuel cells to be configured into a wide array of sizes to fit the required amount of energy desired, whether it be a train, drone, building or car. The electricity produced by a fuel cell then powers a traction motor to drive a vehicle’s wheels, or whatever device you may be powering.
Many governments worldwide are setting mandates and timelines for the adoption of zero emission electric transportation solutions. While consumer preference will determine whether the option is battery or fuel cells, many see fuel cells as the technology of choice for those desiring longer vehicle range capabilities while battery electric vehicles will be ideal as city-cars. Hydrogen fuel cells are seen as the more efficient choice for heavier vehicles such as transit buses or trucks, due to the weight and range constraints of battery-only options.
How does a fuel cell work?
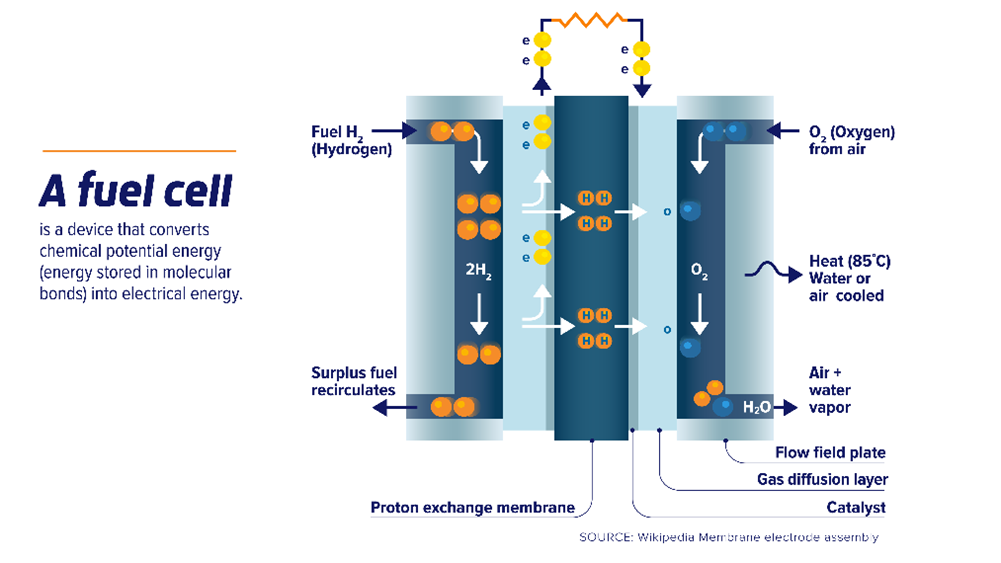
A single fuel cell consists of an electrolyte sandwiched between two electrodes. Oxygen from air passes over one electrode and hydrogen over the other, generating electricity, water and heat. Bipolar plates on either side of the cell help to distribute gases and collect the electrical current.
At the anode, hydrogen is separated into ions and electrons using platinum or similar catalyst. The electrons travel through an external circuit, generating the required amount of power, while the ions pass through the electrolyte to the cathode where, with the help of another catalyst, they join with oxygen atoms to produce water.
Another advantage of fuel cells is that you can make them in your garage. There are dozens of how to make your own hydrogen fuel cell videos on YouTube. The key materials are Platinum, Nafion, Teflon, Silicone Rubber, Graphite, carbon paper and carbon fibre. Nafion can be ordered online and Platinum can be purchased from rare metal dealers.
With Platinum being the most expensive material for use in hydrogen fuel cells a great deal of research has been conducted in finding a less cost effective material. In a 2019 article in Princeton’s website (For hydrogen fuel cells, mundane materials might be almost as good as pricey platinum (princeton.edu) where they state;
For such applications, Platinum’s perfection may not be needed. One good-enough substitute, the researchers found, is hafnium oxyhydroxide that has been treated with a nitrogen plasma (plasma is an ionized gas and is a state of matter found in fluorescent lights and the sun) to incorporate nitrogen atoms into the material.
They also state;
While this hafnium-based film is only about two-thirds as effective as platinum, hafnium is far cheaper than platinum. The researchers plan to test zirconium, which is even cheaper, next.
[2]Other alternatives to platinum for use in hydrogen fuel cells that are being researched are;
- Transition metal nitrites (TMNs)
- Manganese and iron-based metals
- A Cobalt Nitride catalyst
Since it was first invented in 1832 and labelled a curiosity, the hydrogen fuel cell has come a long way. It’s useful and easy to create technology that powers vehicles, aircraft and aiding in the exploration of space.
You can reach Robert Spénard on Twitter Tweet #h20fuelsab
[1] Source About Fuel Cells – CHFCA
[2] Source (New catalysts steer hydrogen fuel cells into mainstream | Cornell Chronicle)

